Immunotherapy has emerged as a promising strategy in cancer treatment, offering the potential for long-term disease control through systemic anti-cancer therapy and immunological memory. However, many patients do not respond to immune checkpoint inhibitors (ICIs) due to the absence of pre-existing anti-cancer immunity. Recent studies have highlighted the potential of mRNA vaccines to bridge this gap, generating anti-cancer immunity and augmenting the effects of ICIs. This article delves into groundbreaking research demonstrating how mRNA vaccines, initially designed for SARS-CoV-2, can also sensitize tumors to ICIs, presenting a paradigm shift in cancer treatment.
This article explores how readily available mRNA vaccines can enhance the efficacy of cancer immunotherapy, focusing on the underlying mechanisms and clinical implications. By examining preclinical models and human data, we uncover how these vaccines stimulate robust anti-tumor immune responses and restore sensitivity in tumors that were previously resistant to ICIs. Furthermore, we will discuss the potential of systemic innate immune modulation as a strategy to sensitize tumors to ICIs, positioning mRNA therapeutics targeting infectious disease antigens as universal modulators of anti-tumor immunity.
The Link Between Innate Immunity and Cancer Treatment
Innate immunity, the body’s first line of defense against pathogens, plays a crucial role in cancer development and treatment. Immune checkpoint inhibitors (ICIs) have shown remarkable success in treating various cancers by blocking proteins that suppress the immune system. However, ICIs are often ineffective in patients without pre-existing immunity. Recent research indicates that SARS-CoV-2 mRNA vaccines can bridge this gap by enhancing innate immune responses, particularly increasing type I interferon levels. This increase enables innate immune cells to prime CD8+ T cells, which are critical for targeting tumor-associated antigens.
A study published in Nature highlights this phenomenon, stating, “In preclinical models, SARS-CoV-2 mRNA vaccines led to a substantial increase in type I interferon, enabling innate immune cells to prime CD8+ T cells that target tumour-associated antigens.” This suggests that mRNA vaccines can transform immunologically ‘cold’ tumors into ‘hot’ tumors, making them more susceptible to ICIs. The concurrent use of ICIs is essential to maximize efficacy, as these tumors respond by increasing PD-L1 expression, which can otherwise dampen the immune response.
The Mechanism: How mRNA Vaccines Sensitize Tumors
The process through which mRNA vaccines sensitize tumors involves several key steps. First, the mRNA vaccines stimulate a surge in antiviral cytokines, including IFNα, which drives systemic innate immune activation. Tumor-resident innate immune cells, activated by this cytokine surge, prime T cells. These T cells become activated, infiltrate tumors, and initiate the killing of tumor cells. However, tumor cells evade this attack by upregulating PD-L1 expression. Concomitant treatment with ICIs enables the mRNA vaccines to overcome this compensatory response, leading to tumor regression and improved survival. This multi-step process underscores the complex interplay between innate and adaptive immunity in cancer treatment.
According to the Nature article, “mRNA vaccines first stimulate a surge in antiviral cytokines, including IFNα, that drive systemic innate immune activation. Tumour-resident innate immune cells activated by this cytokine surge prime T cells, which become activated and infiltrate tumours.” This highlights the vaccine’s initial impact on the innate immune system and its subsequent effects on adaptive immunity.
Clinical Evidence: Human Trials and Improved Survival Rates
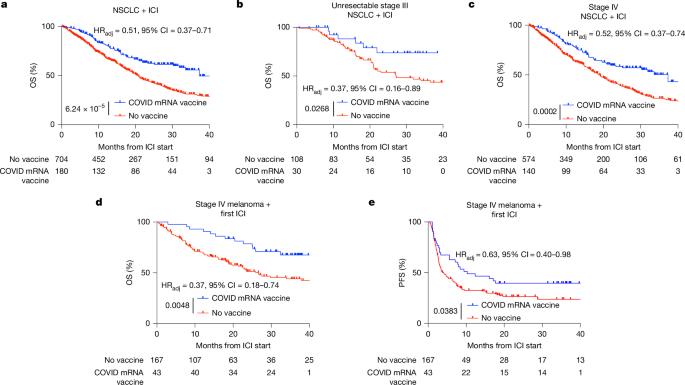
Clinical trials have provided compelling evidence supporting the efficacy of mRNA vaccines in sensitizing tumors to ICIs. Studies involving patients with non-small cell lung cancer (NSCLC) and melanoma have demonstrated that receiving SARS-CoV-2 mRNA vaccines within 100 days of initiating ICI treatment is associated with significantly improved median and three-year overall survival. This benefit is observed even in patients with immunologically cold tumors, suggesting that mRNA vaccines can restore immune sensitivity in previously resistant cases. These findings indicate a promising new avenue for enhancing cancer treatment outcomes.
The Nature study reports, “receipt of SARS-CoV-2 mRNA vaccines within 100 days of initiating ICI is associated with significantly improved median and three-year overall survival in multiple large retrospective cohorts. This benefit is similar among patients with immunologically cold tumours.” This data underscores the potential of mRNA vaccines to transform cancer treatment by improving patient survival rates.
Preclinical Models: Validating the Effects in Animals
To validate the clinical findings, researchers have utilized preclinical models to replicate the effects of mRNA vaccines on tumor sensitization. These models involve administering commercial preparations of COVID mRNA vaccines to tumor-bearing animals in conjunction with ICIs. The results consistently show that this combination is superior to either monotherapy alone. Specifically, mice with established B16F0 tumors and Lewis lung carcinoma (LLC) exhibit significant tumor regression and reduced metastatic lesions when treated with the combination of mRNA vaccines and ICIs.
The article highlights, “In preclinical models, SARS-CoV-2 mRNA vaccines led to a substantial increase in type I interferon, enabling innate immune cells to prime CD8+ T cells that target tumour-associated antigens.” These preclinical results confirm the mechanistic insights observed in clinical studies, reinforcing the potential of mRNA vaccines as a synergistic treatment approach.
Type I Interferon: A Key Mediator of Immunity
Further investigation into the mechanisms underlying tumor sensitization has revealed the critical role of type I interferon (IFN). By blocking the IL-1 and IFNα receptors, researchers have found that anti-tumor responses are completely abrogated when blocking type I interferon signaling with IFNAR1 monoclonal antibodies. This underscores the importance of IFN in driving both innate and adaptive immunity. Direct administration of type I IFN has been shown to recapitulate the anti-tumor effects, highlighting its central role in the observed therapeutic benefits.
As noted in the study, “anti-tumour responses were completely abrogated when blocking type I interferon signalling with IFNAR1 monoclonal antibodies… Moreover, direct administration of supraphysiologic doses of type I IFN recapitulated the antitumour effects.” This demonstrates that Type I IFN stimulation is a critical target for cancer treatment.
Clinical Implications and Future Directions
The findings presented in this article have significant implications for cancer treatment strategies. The demonstration that clinically available mRNA vaccines targeting non-tumor-related antigens can sensitize tumors to ICIs opens new avenues for improving treatment outcomes. As personalized neoantigen vaccines require considerable manufacturing time, off-the-shelf RNA-LNPs targeting tumor-associated or even infectious disease antigens may represent widely available, low-cost alternatives. Further research is needed to explore the optimal timing, dosage, and combination strategies for mRNA vaccines and ICIs in various cancer types.
According to the authors, “off-the-shelf RNA-LNPs targeting tumour-associated or even infectious disease antigens may represent widely available, low-cost alternatives for patients waiting for personalized neoantigen vaccines or in settings in which personalized neoantigen vaccines are not available.” These widely accessible mRNA vaccines could serve as universal modulators of anti-tumor immunity.
Conclusion: Revolutionizing Cancer Immunotherapy with Readily Available Vaccines
The discovery that SARS-CoV-2 mRNA vaccines can sensitize tumors to immune checkpoint blockade marks a significant advancement in cancer immunotherapy. By stimulating robust anti-tumor immune responses, these vaccines offer a new means of overcoming resistance to ICIs, even in immunologically cold tumors. The underlying mechanism involves a surge in antiviral cytokines, particularly type I interferon, which drives systemic innate immune activation and primes T cells for tumor infiltration and destruction.
The clinical evidence, supported by preclinical models, underscores the potential of readily available mRNA vaccines to improve survival rates and treatment outcomes in patients with various cancers. This research not only highlights the therapeutic benefits of repurposing existing vaccines but also paves the way for developing universal mRNA therapeutics specifically designed to reset patient immune systems for enhanced response to immunotherapy. The integration of mRNA vaccines into cancer treatment regimens promises to revolutionize the field, offering new hope and improved prognoses for patients worldwide. As the field advances, future research should focus on optimizing the use of these vaccines to maximize their synergistic effects with existing cancer therapies, ultimately transforming the landscape of cancer care.
Leave a Reply